
This article was published on Arbona Health Hub Volume 1 Issue 1 (ISSN: 3065-5544).
Have you ever wondered how drugs work to combat neurodegenerative diseases like Alzheimer’s? Welcome back to the second part of this exploration, where we delve deeper into the intricate mechanisms and pharmaceutical options related to Alzheimer’s Disease.
Alzheimer’s Disease (AD) is the most common form of neurodegenerative disease and poses a significant public health challenge worldwide. Despite decades of research, there is still no cure for AD, making prevention the primary focus.
In today’s article, we’ll peel back the layers to reveal the science behind Alzheimer’s treatments. Whether you’re a medical professional, researcher, or someone affected by this condition, this article aims to provide valuable insights into the latest advancements in Alzheimer’s treatment strategies.
We’ll explore the drugs available in pharmacies, and the treatments they offer. Don’t worry, we’ll keep things simple for easy understanding.
Before we dive in, I want to express my gratitude to Jessica Tang, a medical student at the University of California, Davis School of Medicine, for her valuable contributions to today’s article. Thanks, Jessica, for sharing your expertise with us.
Overview of FDA-Approved Medications
In the realm of AD treatment, the pharmaceutical industry offers a range of therapeutic options targeting different aspects of the condition. While medications for AD do not offer a cure, it’s significant to recognize that they can enhance the quality of life and extend periods of independence for individuals affected by the condition.
Currently, the Food and Drugs Administration (FDA) has given the green light to various drugs designed to tackle different symptoms of Alzheimer’s disease as it progresses through its various stages. These stages – mild, moderate, and severe – are determined by how well someone does on tests that check memory, understanding of time and location, and ability to think and reason, mostly falling under the physical and neurological exam. We cannot leave behind that these tests are accompanied by brain scans such as magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET), as well as other biomarkers such as blood tests, cerebrospinal fluid tests, and genetic tests.
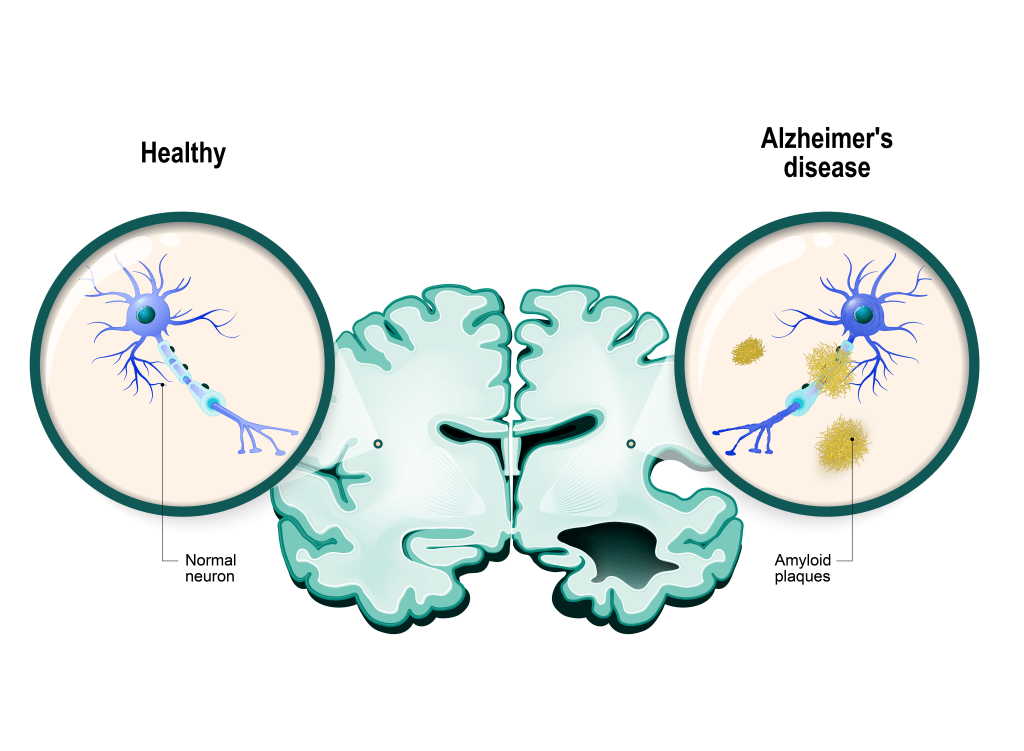
Monoclonal antibodies, such as Aducanumab and Lecanemab, represent a significant advancement as the first disease-modifying therapies for AD. Amyloid-beta (A-β) plaques are not cleared by the body as a consequence of the development of AD. Aducanumab primarily targets the A-β plaques for clearance, while Lecanemab exhibits a higher affinity for protofibril A-β, which eventually aggregate to form the A-β plaques.
Additionally, drugs like Donepezil, Galantamine, and Rivastigmine function by inhibiting acetylcholinesterase, an enzyme responsible for breaking down acetylcholine. By doing so, these drugs increase the concentration of acetylcholine at synapses, aiming to improve the symptoms associated with AD. These diverse treatment approaches offer hope for alleviating the burden of AD and improving the quality of life for affected individuals.
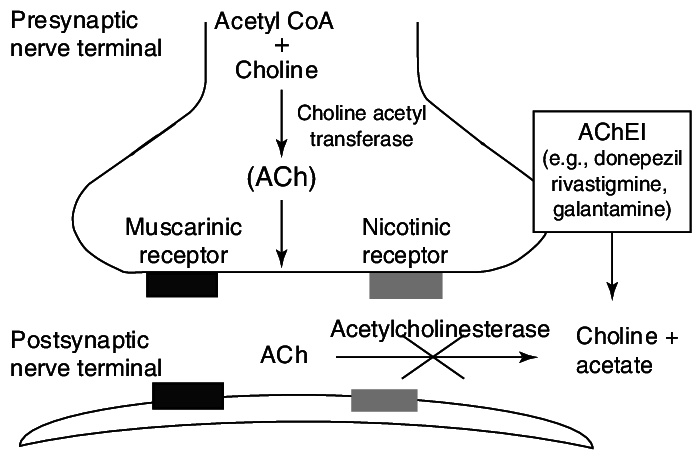
I know this image might look complicated, even the pronunciation is hard… but, this is the simplest explanation of how these acetylcholinesterase inhibitors works and their mechanism of action.
In addition to the pharmaceutical industry’s efforts in Alzheimer’s treatment, ongoing research is shedding light on novel approaches and insights. We can see hundreds or even thousands of ongoing research dedicated to finding better understanding and possible drugs that can help for this and many neurological diseases.
During my conversation with Jessica Tang, she highlighted key aspects of her research. Her studies were centered on AD within the context of tauopathies, which are characterized by abnormal accumulation of tau protein aggregates. Specifically, she investigated the effects of Fyn Kinase inhibition on a mouse model of tauopathy. Her findings suggested that inhibiting Fyn Kinase could potentially reduce phospho-Tau accumulation in the brain, offering a promising avenue for further exploration.
However, recent developments, such as the discontinuation of Adacanumab (published on January 31st of 2024 by Biogen) , have prompted shifts in Alzheimer’s research and treatment strategies. The decision to limit coverage of future anti-amyloid therapies by the Center for Medicare and Medicaid Services has redirected researchers’ focus toward alternative drug targets and mechanisms. This includes exploring treatments that target inflammation, alongside continued investigation into amyloid and tau-related therapies.
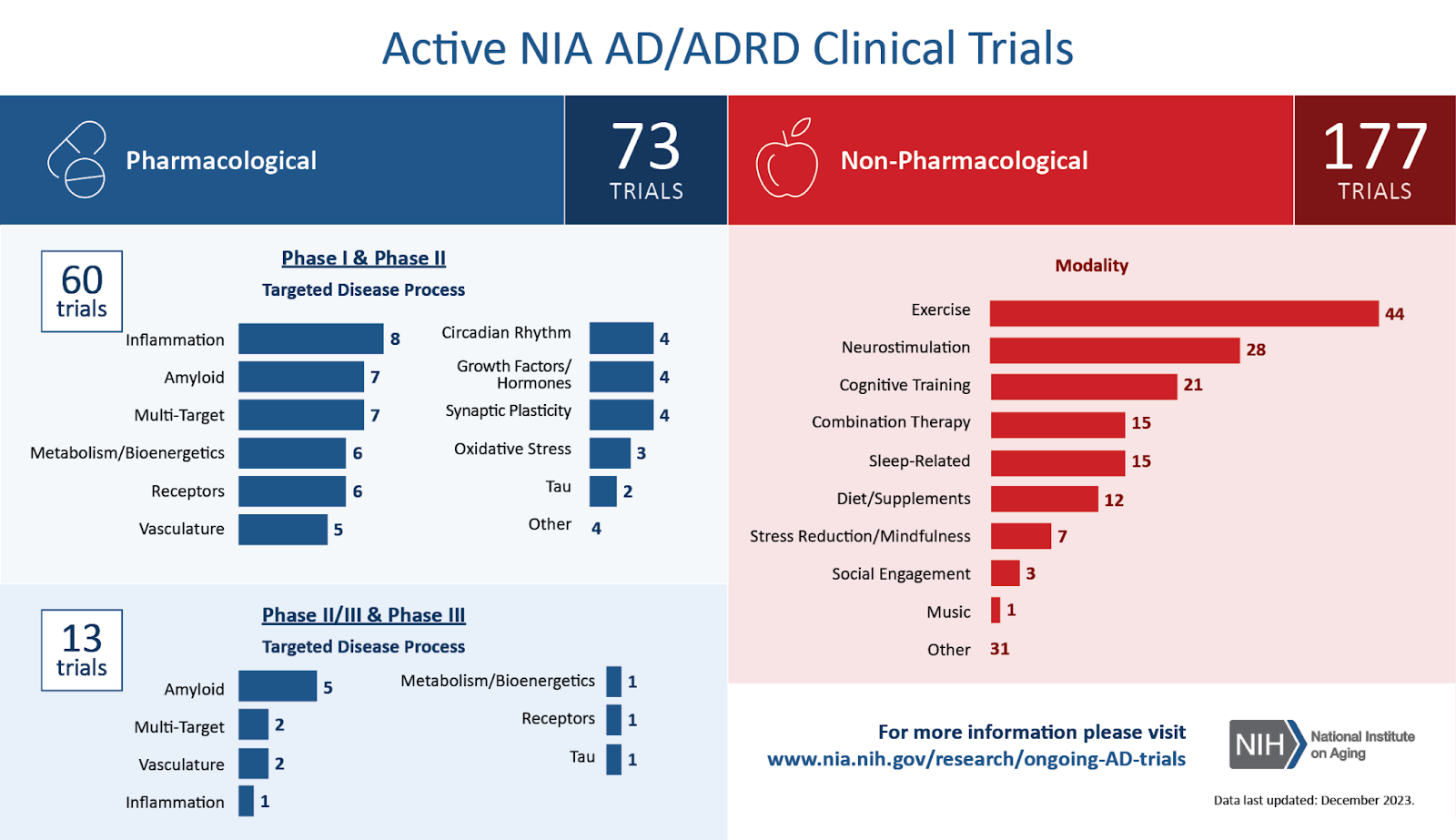
Despite advancements, challenges persist in developing effective treatments for Alzheimer’s disease. These challenges include the need to diversify drug targets and mechanisms beyond amyloid and tau. Looking ahead, non-pharmacological interventions and multidisciplinary approaches hold promise in slowing disease progression.
I hope you enjoyed this “simple” breakdown of this complex disease. The amount of research behind this and many neurological diseases in order to explain how they develop and attack our body is still far from our understanding and with big limitations since diagnosing them poses a challenge, and medications face obstacles in reaching the brain due to the brain’s distinct blood supply, which is largely isolated from the rest of the body. But with this information, you can now understand a little bit more that we, the scientific community, are working toward finding answers without rest. Together, let’s continue unraveling the mysteries of the brain, one discovery at a time!
References
- Tang, S. J., Fesharaki-Zadeh, A., Takahashi, H., Nies, S. H., Smith, L. M., Luo, A., Chyung, A., Chiasseu, M., & Strittmatter, S. M. (2020). Fyn kinase inhibition reduces protein aggregation, increases synapse density and improves memory in transgenic and traumatic Tauopathy. Acta neuropathologica communications, 8(1), 96.
- https://knowablemagazine.org/content/article/health-disease/2020/seeking-better-test-alzheimers
- Chowdhury, S., & Chowdhury, N. S. (2023). Novel anti-amyloid-beta (Aβ) monoclonal antibody lecanemab for Alzheimer’s disease: A systematic review. International journal of immunopathology and pharmacology, 37, 3946320231209839. https://doi.org/10.1177/03946320231209839
- https://www.nia.nih.gov/research/ongoing-AD-trials
- Zhang, Y., Chen, H., Li, R., Sterling, K., & Song, W. (2023). Amyloid β-based therapy for Alzheimer’s disease: challenges, successes and future. Signal transduction and targeted therapy, 8(1), 248. https://doi.org/10.1038/s41392-023-01484-7
- https://www.researchgate.net/publication/233623210_Use_of_acetylcholinesterase_inhibitors_in_Alzheimer’s_Disease
- https://investors.biogen.com/news-releases/news-release-details/biogen-realign-resources-alzheimers-disease-franchise


[…] for cancer detection. PET scans can also be used to detect heart disease and brain disorders like Alzheimer’s and […]
LikeLike